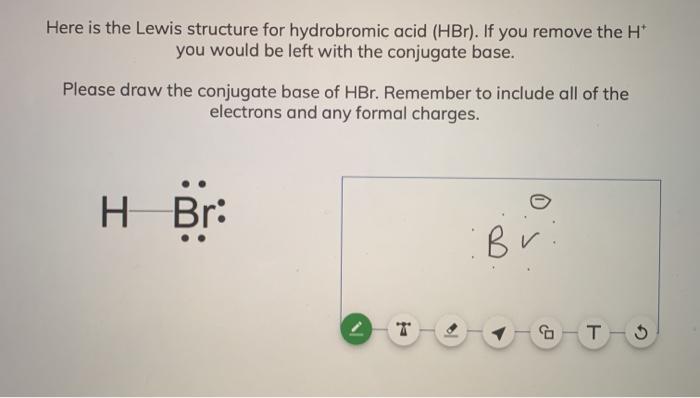
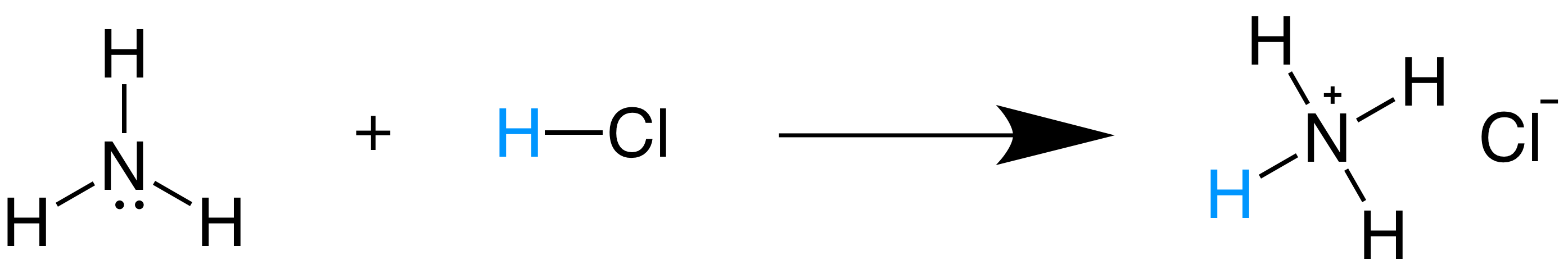SOLVED: What is the conjugate base of HBr? Br+ B Br D: BrOH QUESTION 6 Which compound in the following pair is the stronger acid? CHBCHZCH3 (pKa = 50) and CHBCHZOH (pKa =

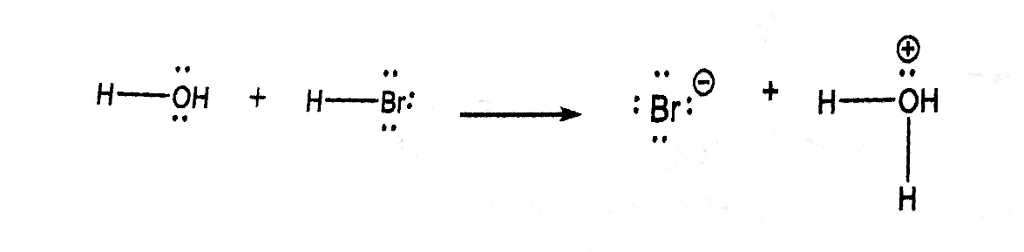
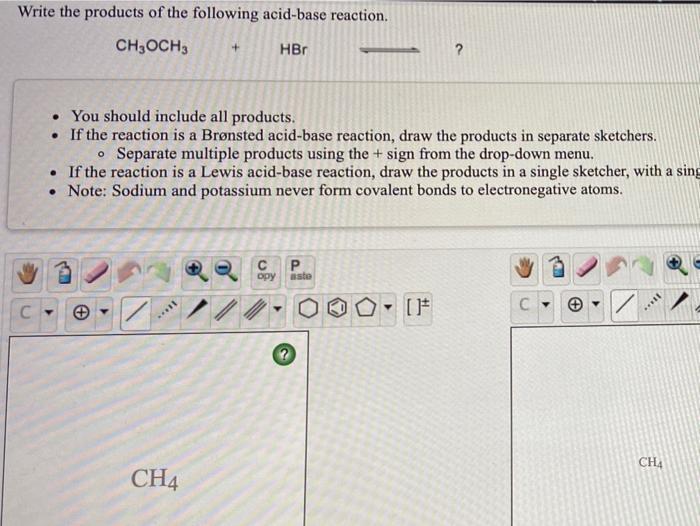
PHILIPPINE CHEMISTRY PROFESSIONALS BOARD EXAM REVIEWER - Which one of the following mechanistically depicts the acid-base reaction that occurs when hydrobromic acid(HBr) is added to methanol (CH4O)? Please refer to attached image.

1 Introduction to Acids and Bases The earliest definition was given by Arrhenius: An acid contains a hydrogen atom and dissolves in water to form a hydrogen. - ppt download
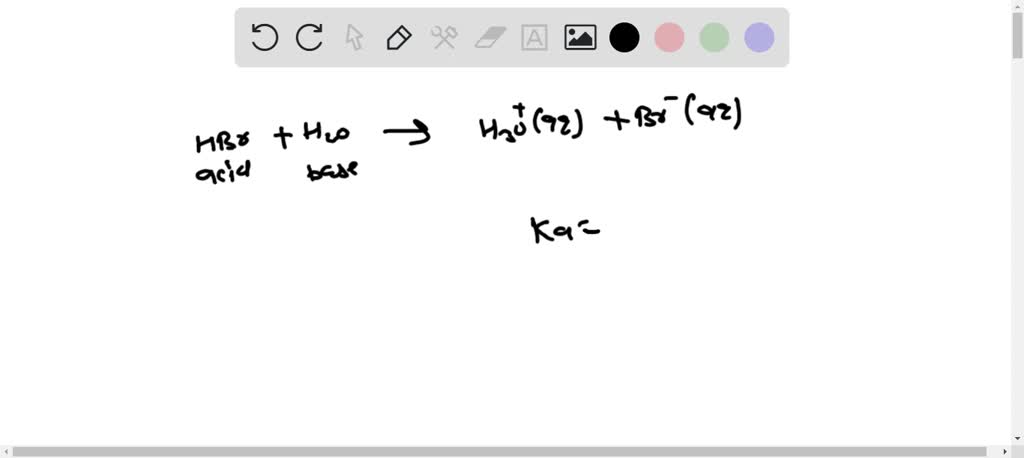
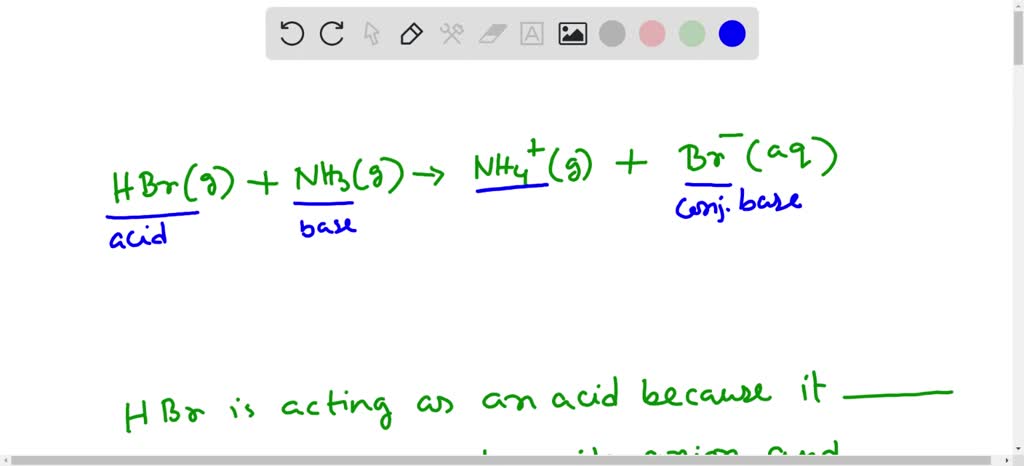
SOLVED: (a) write the net acid-base reaction that occurs when HBr is added to water. (use the lowest possible coefficients. Omit states-of-matter in your answer.)(b) What is the relative Ka value for
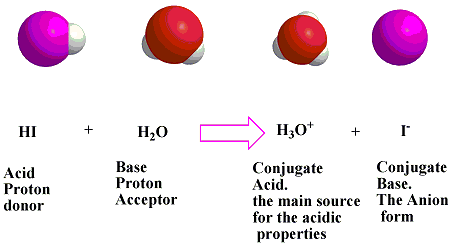
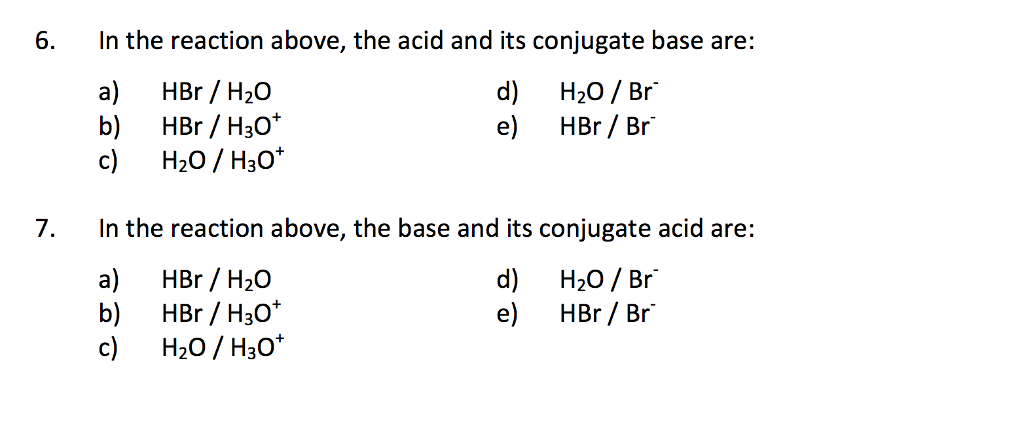
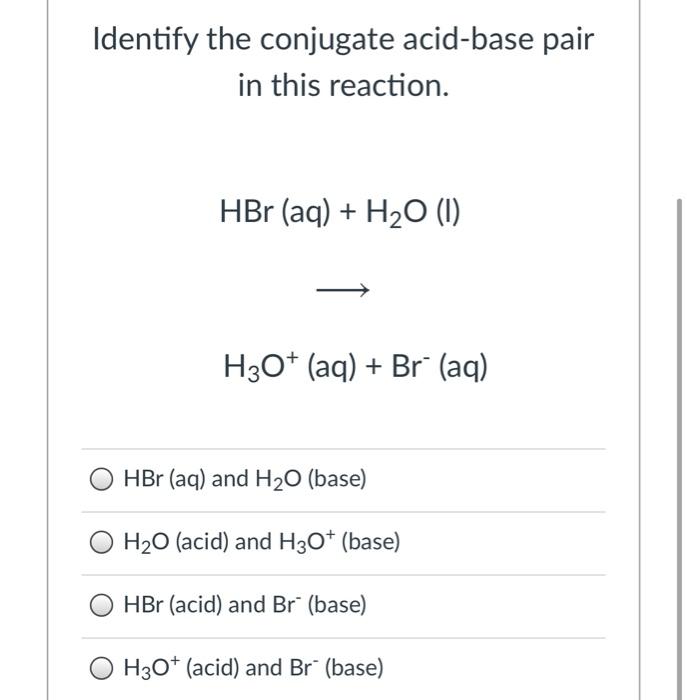
Which pair is a Brønsted–Lowry conjugate acid–base pair? NH_3 ; NH_4^+ or H_3O^+ ; OH^- or HCl; HBr or ClO_4^(-); ClO_3^- | Socratic
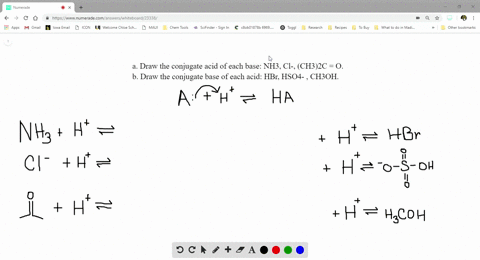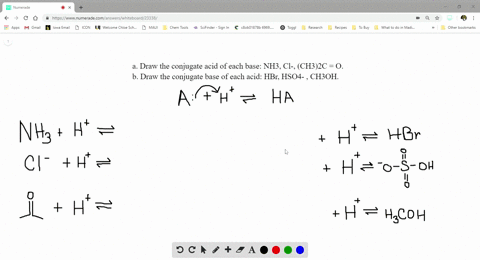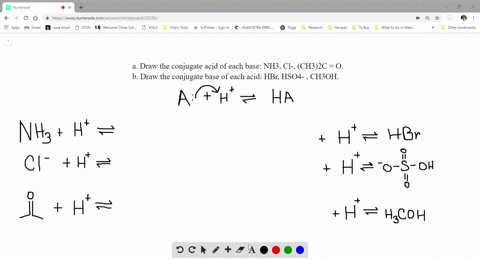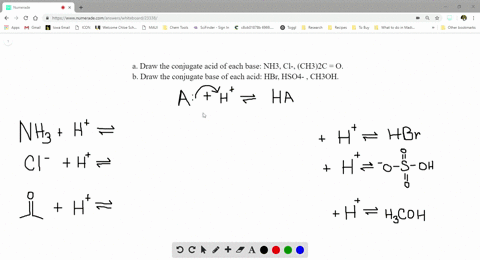
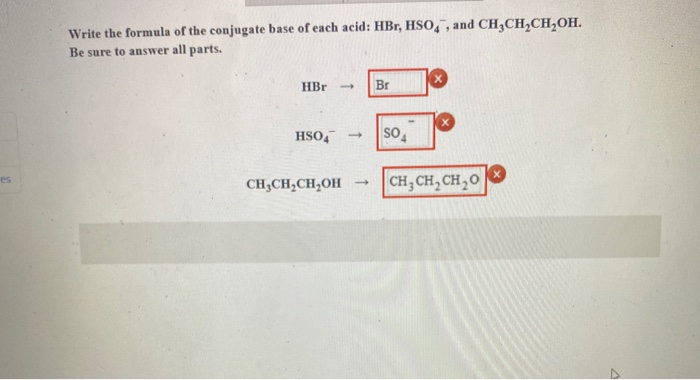
SOLVED:a. Draw the conjugate acid of each base: NH3, Cl^-, (CH3)2C = O. b. Draw the conjugate base of each acid: HBr, HSO4^- , CH3OH.